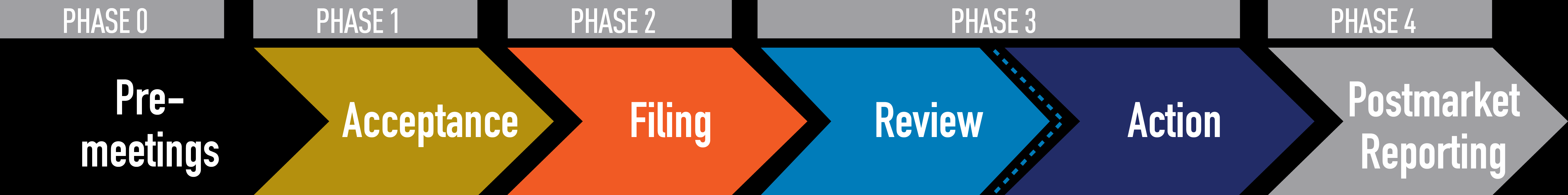
The FDA PMTA (Pre-market Tobacco Product Application) process refers to the requirement for manufacturers to submit an application to the U.S. Food and Drug Administration (FDA) for approval of any new tobacco products (including e-cigarettes and vaping devices) before they can be marketed. This process ensures that products meet the standards for public health protection.
Table of Contents
Ongoing Reviews and Denials:
-
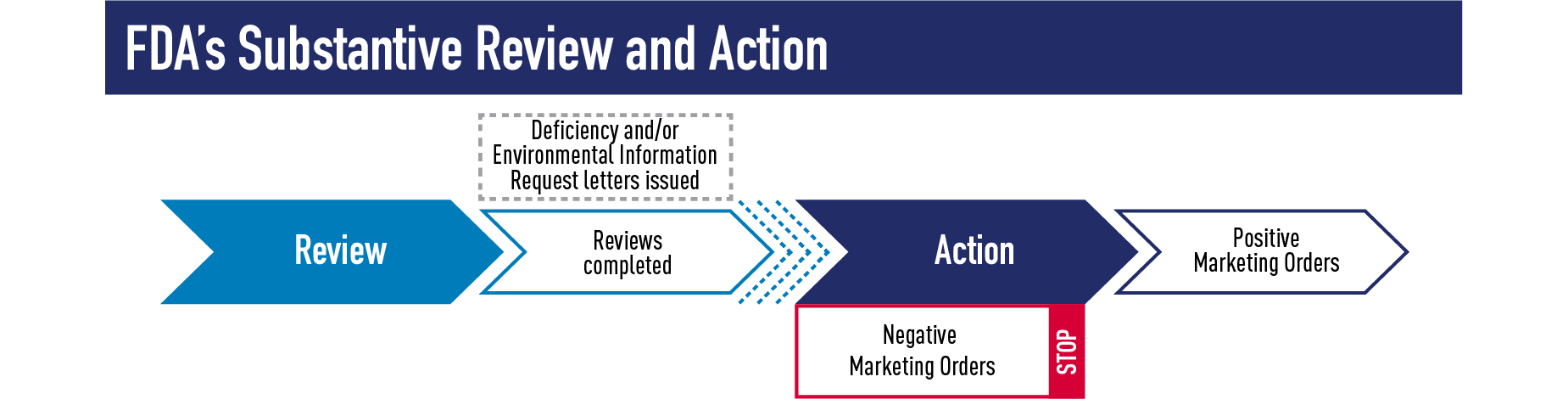
- The FDA continues to review PMTAs for various vaping products. As of recent updates, some manufacturers have had their applications rejected or denied due to insufficient data demonstrating that the products meet the required public health standards.
- A notable update is the denial of thousands of PMTAs for certain flavored e-cigarettes and other vaping devices. However, companies with approved applications can continue to market their products.
Focus on Youth Appeal:
-
- The FDA’s primary concern in reviewing PMTAs is the potential appeal of these products to underage users. The agency has been particularly focused on flavored e-cigarettes and nicotine salts that might attract younger audiences.
- Manufacturers are increasingly required to submit robust data that proves their products do not promote or encourage youth use.
FDA PMTA Legal Challenges:
-
- Some companies have challenged FDA decisions in court, arguing that the agency’s denials or delays in processing applications violate the law. These legal battles are ongoing, and some manufacturers have been granted temporary approvals while the courts deliberate on the matter.
Extension for Some Products:
-
- Some vaping products have been granted temporary extensions while awaiting further data submission or FDA decisions. However, the FDA continues scrutinizing products that do not meet public health standards.
FDA PMTA Mandated Warning Labels:
-
- Products granted PMTA approval are subject to the FDA’s warning label requirements, which include a statement that the product contains nicotine and that nicotine is an addictive chemical.
 Key Takeaways:
Key Takeaways:
- Manufacturers are still required to submit PMTAs for all new tobacco products (including e-cigarettes), and many products are being denied or temporarily blocked.
- Flavored products and products that appeal to youth are receiving heightened scrutiny.
- Legal challenges to the FDA’s decisions may continue to shape the outcome of the PMTA process.