The global vape market is currently undertaking a more stringent regulatory landscape. Nations like the United States, Latvia, and Thailand have introduced new regulations, demonstrating their commitment to balancing public health concerns with market growth.
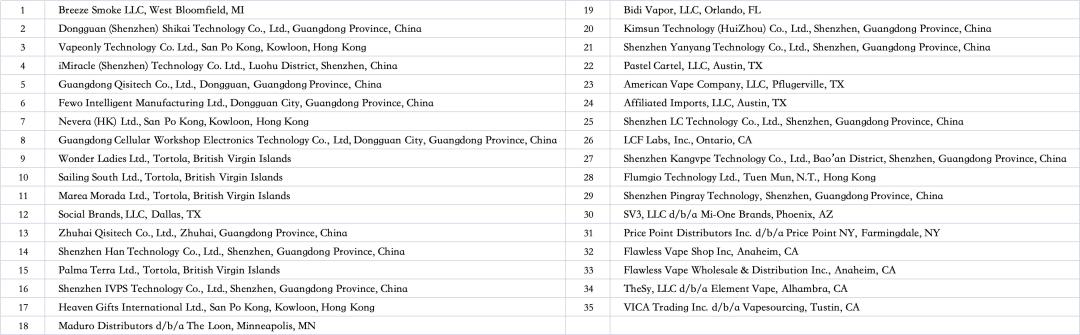
List of 35 accused companies
On July 17, the United States International Trade Commission (USITC) announced the initiation of a 337 investigation into specific disposable e-cigarette devices. This investigation was prompted by a complaint from a Reynolds American subsidiary, which accused 35 parties of infringing on its patent rights. The USITC is expected to establish a deadline for the investigation within 45 days, and should it find any violations, it could issue an order to prohibit the import and sale of the infringing products.
In a related development, the U.S. Food and Drug Administration (FDA) granted marketing authorization for British American Tobacco’s Vuse Alto product line on July 18.
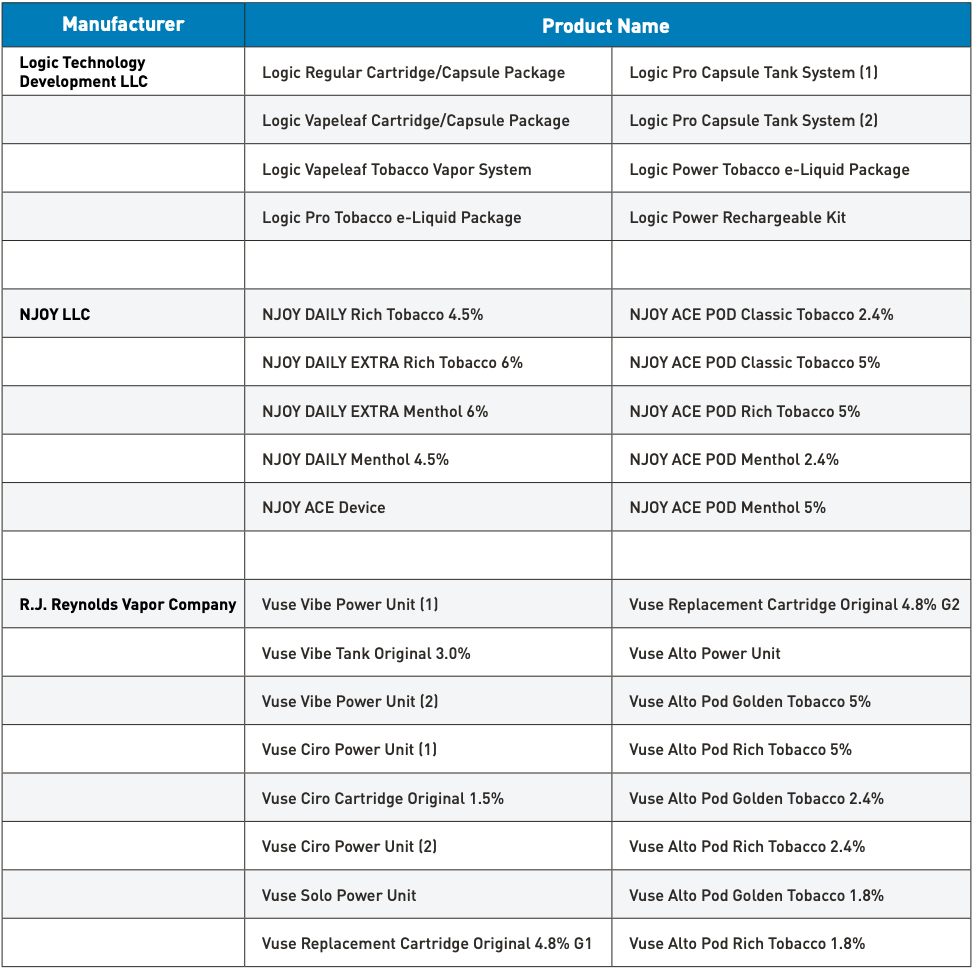 The authorized vape brands by the FDA for sale in the United States
The authorized vape brands by the FDA for sale in the United States
In Europe, Latvia has imposed fines of 50,000 euros each on two vape companies for violating advertising bans on social media, which negatively affected teenagers.
Meanwhile, the Thai government has intensified its efforts against e-cigarettes by forming a special task force. New measures include prohibiting e-cigarette use in public areas and shutting down 309 online sales accounts. Thailand has classified e-cigarettes as illegal, with offenders facing potential prison sentences of up to 10 years.
The Vape Market is facing more challenges
These global regulatory developments present both challenges and opportunities for the vape industry. Moving forward, the industry will need to prioritize product safety and compliance while striving to balance the needs of adult smokers with the protection of youth.






